
SARAH ELIZABETH PAGE
Art & Design


© Sarah Elizabeth Page 2013



Familial Breast Cancer Program, 2011-12
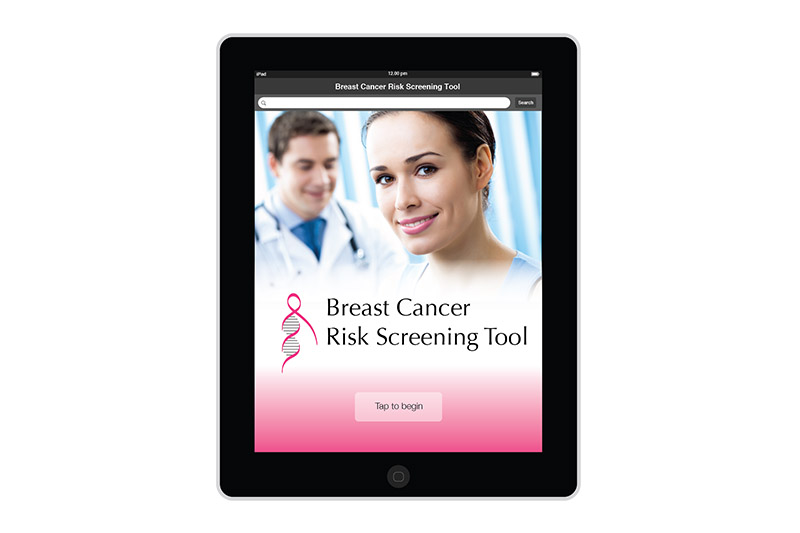
The logo design was created in September 2011 for the Familial Breast Cancer Program at the University of Illinois. The group offers services and information to patients that are at risk for breast cancer. In the design, I combined a DNA double helix with the ubiquitous pink ribbon.
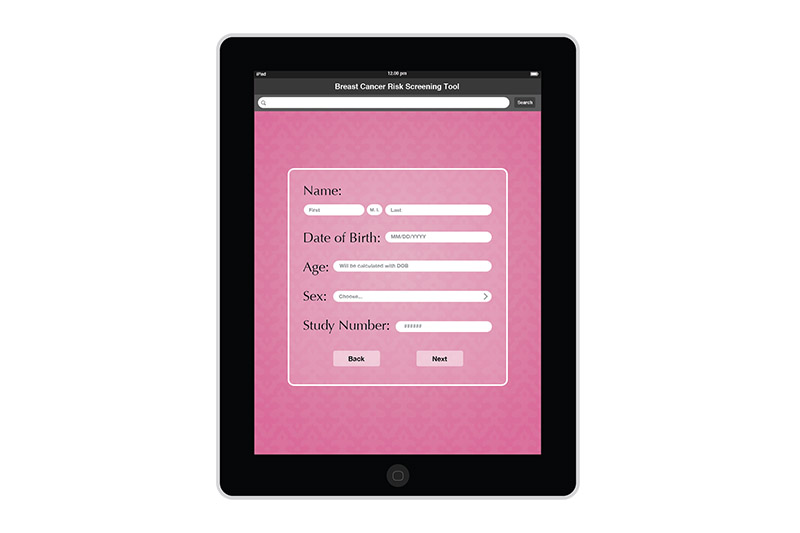
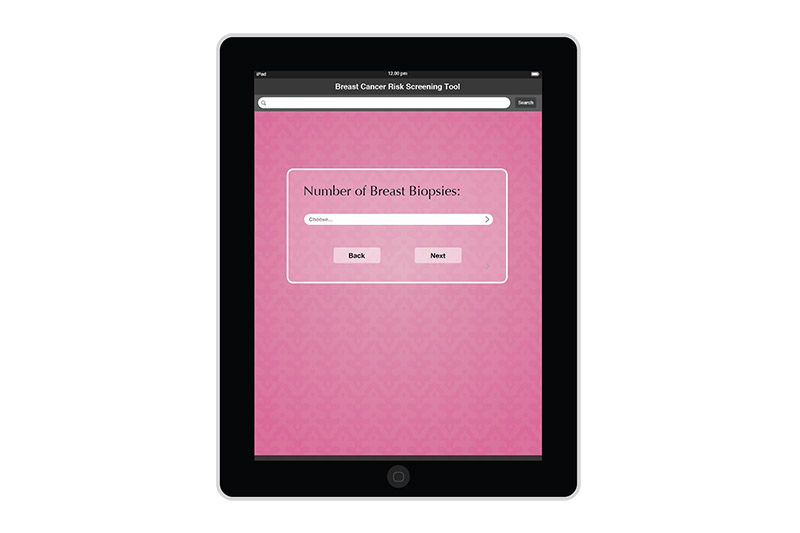
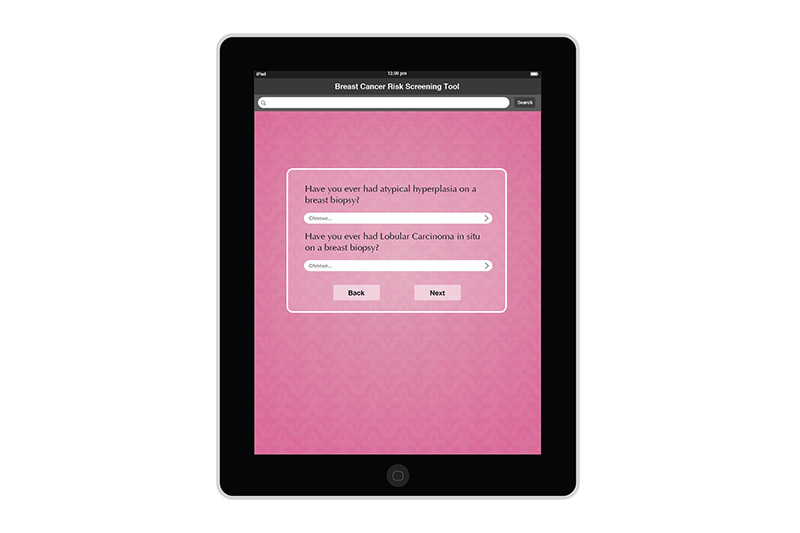
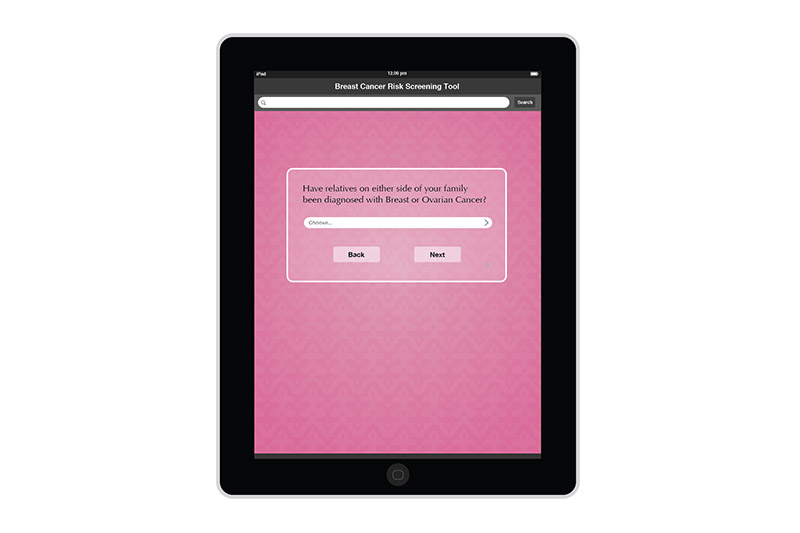
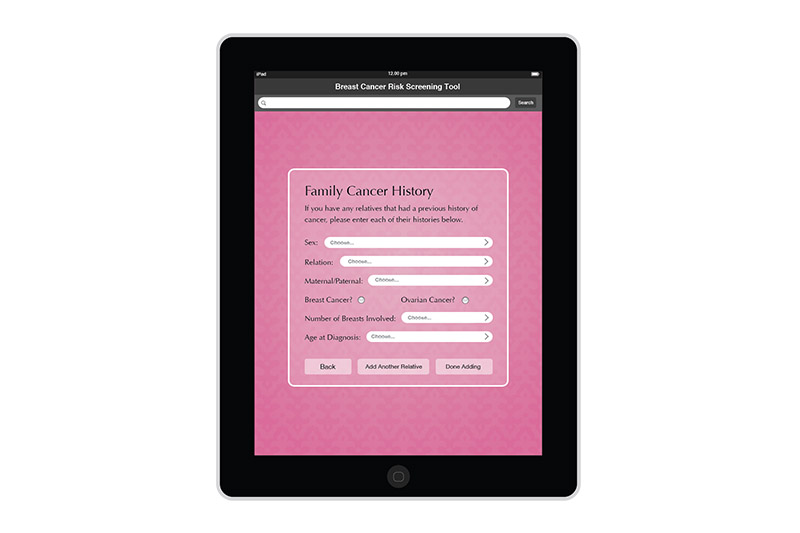
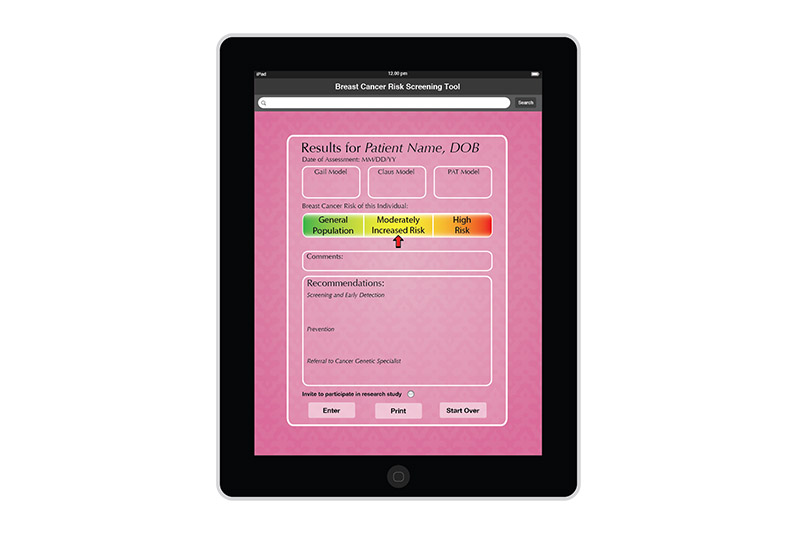
I created the design for the Breast Risk Assessment Tool iPad application during the spring of 2012 at the University of Illinois at Chicago. The application was a part of a study to assess the risk of breast cancer in the general population. Data is entered in a few pages. After calcualations are made, three different risk scores are shown on a results page. The patient's risk is also graphically shown on a risk scale. Comments and recommendations for the patient are also reflected. The results page then allows the practitioner to invite the patient to enter a more involved study or not.